

#Tetra chemistry free
These results clearly demonstrate that the CBPQT 4+ ring can cross both an MPTTF 2+ and a TTF 2+ electrostatic barrier and that the free energy of activation required to cross MPTTF 2+ is ca.
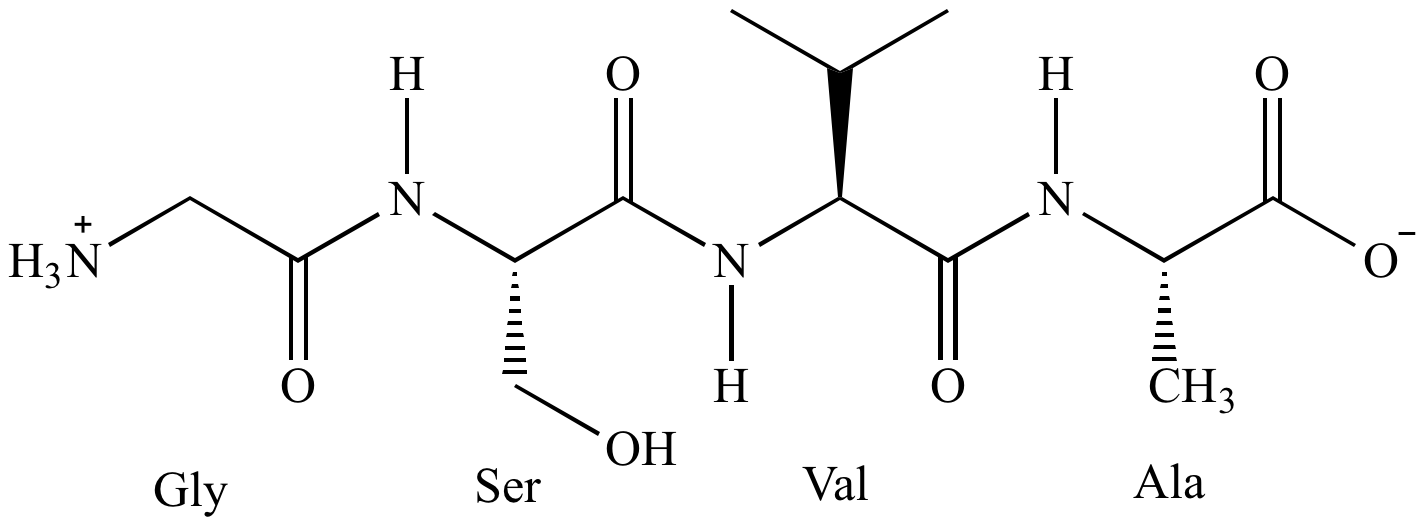
To learn more about all aspects of fishkeeping, we recommend joining us on Facebook, signing up for TetraCare and, when you have a question, calling our Contact Us at 80. 1H NMR spectroscopy carried out in CD 3CN at 298 K on a chemically oxidized sample of 1⋅MPTTF 4+ revealed that the metastable state 1⋅TEG 8+ is only short-lived with a lifetime of a few minutes and it was found that 70 % of the positively charged CBPQT 4+ ring moved from 1⋅TEG 8+ to the HQ station, while 30 % moved to the much weaker OP station. Saltwater aquariums require more advanced types of filtration, an understanding of saltwater chemistry, and special feeding and care regimens. Following tetra-oxidation of 1⋅MPTTF 4+, a high-energy state of 1 8+ was obtained (i.e., 1⋅TEG 8+) in which the CBPQT 4+ ring was located on the TEG linker connecting the di-oxidized TTF 2+ and MPTTF 2+ units. They have special names that are beyond the scope of this book. The electrochemical studies showed that CBPQT 4+ in 1⋅MPTTF 4+ undergoes ring translation as result of electrostatic repulsion from the oxidized MPTTF unit. Substances with carbon and hydrogen are organic compounds. The spectroscopic data revealed that the majority (77 %) of the tetra-stable rotaxane 1 4+ exist as the translational isomer 1⋅MPTTF 4+ in which the CBPQT 4+ ring encircles the MPTTF station. The rotaxane was characterized in solution by 1H NMR spectroscopy and cyclic voltammetry. The TTF and the MPTTF stations are located in the middle of the dumbbell component and are connected by a triethylene glycol (TEG) chain in such a way that the pyrrole moiety of the MPTTF station points toward the TTF station, while the TTF and MPTTF stations are flanked by the OP and HQ stations on their left hand side and right hand side, respectively. The dumbbell component is comprised of an oxyphenylene (OP), a tetrathiafulvalene (TTF), a monopyrrolo-TTF (MPTTF), and a hydroquinone (HQ) unit, which can act as recognition sites (stations) for the tetra-cationic cyclophane cyclobis(paraquat- p-phenylene) (CBPQT 4+). R.A tetra-stable donor–acceptor rotaxane 1⋅4PF 6 has been synthesized. Contact of TBAF with acid liberates toxic gas.ġ) Patent Reference: WO2015140133, page 103, (11.7 MB)Ģ) Patent Reference: WO2014152144, page 59, (4.6 MB)ģ) Patent Reference: WO2016014463, page 101, (6.7 MB)Ĥ) Patent Reference: WO2016011390, page 151, (20.2 MB)ĥ) Wikipedia: Tetra- n-butylammonium fluoride ( link)Ħ) Tetrabutylammonium fluoride solution ( link)ħ) Pearson, A. Ī mixture of the SM (150 mg, 0.36 mmol), TBAF (281 mg, 1.08 mmol), TMSCN (107 mg, 1.08 mmol) in DMF (3 mL) was stirred at 100 C for 1 h. The mixture was cooled to 0 C and treated with TBAF. Reagent in substitution reactions with TMSCNĪ solution of the SM (80.0 g, 286 mmol) and TMSCN (28.2 g, 286 mmol) in ACN (600 mL) was stirred at RT for 30 min. Upon completion, the resulting solution was. Item Details: Tetra Tech (RTW) Water Chemistry, Process, and Corrosion Control Model performs useful calculations for water treatment processes. Ī mixture of the SM (319 mg, 0.848 mmol) and TBAF (221 mg, 0.848 mmol) in THF (20 mL) was stirred at RT overnight. The reaction mixture was stirred at 80 C for 12 h. To a stirred solution of the SM (250 mg, 0.43 mmol) in THF (4 mL) was added TBAF (4 mL, 1M in THF). TBAF will always contain some water due to the fact that the fluorine ion is such a great hydrogen bond acceptor. TBAF is typically obtained as a trihydrate (TBAF-3H2O), 1M solution in THF, or 75 wt% solution in H2O. Reactions involving TBAF are typically carried out at temperatures below 100 C due to its low thermal stability. The good solubility of TBAF in organic solvents makes it a useful alternative to poorly soluble inorganic bases. The fluorine anion is typically used for deprotection of silyl ether groups or as a mild base. TBAF is a quaternary salt that is used as a source of fluorine. Molecular Weight: 261.46 g/mol (anhydrous basis) Concise Chemistry Part II - Selina Solutions for Class Chemistry ICSE Chapter 12: Get free access to Organic Chemistry Class Solutions which includes all.


 0 kommentar(er)
0 kommentar(er)
